Technologies & Services
Your Full Solution Provider
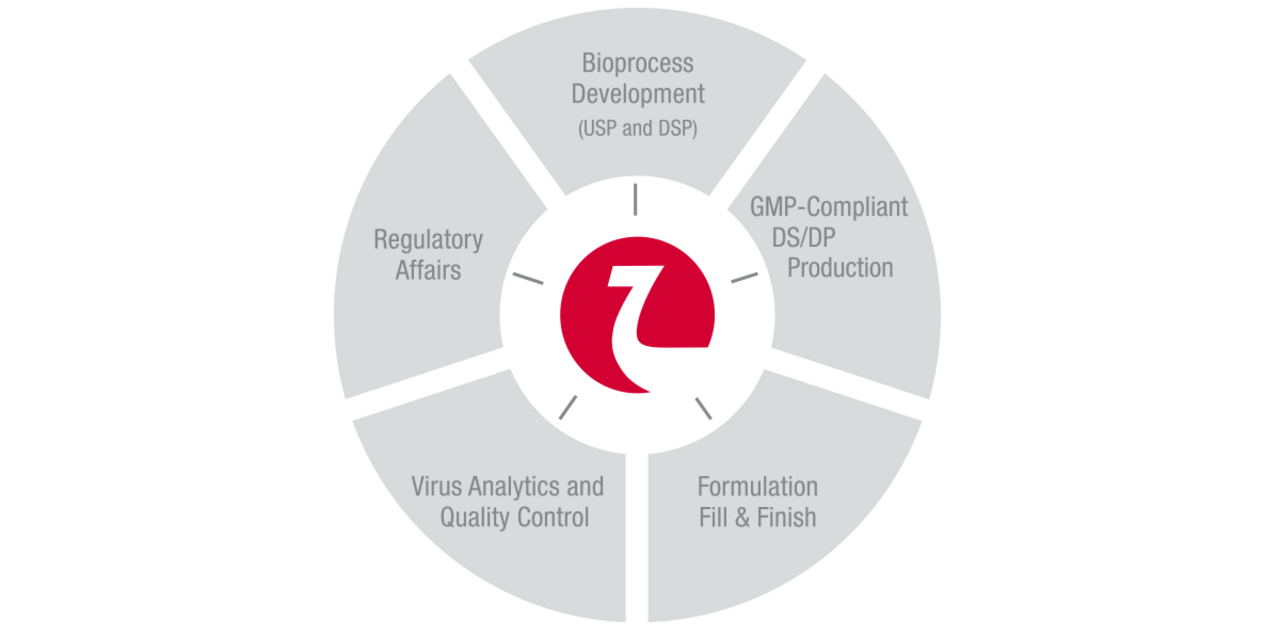
Nordmark Biotech offers you the entire value chain from process development to market supply.
In accordance with cGMP we produce active ingredients and finished pharmaceutical products for well-known pharmaceutical companies and our own sales organisation.
Our service package is completed by pharmaceutical and analytical process development, stability studies and worldwide regulatory support.